
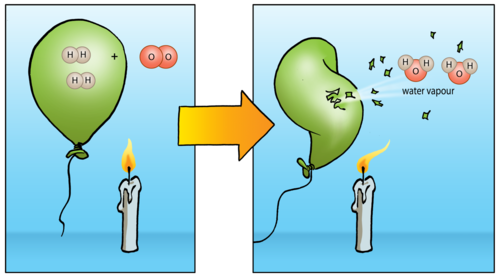
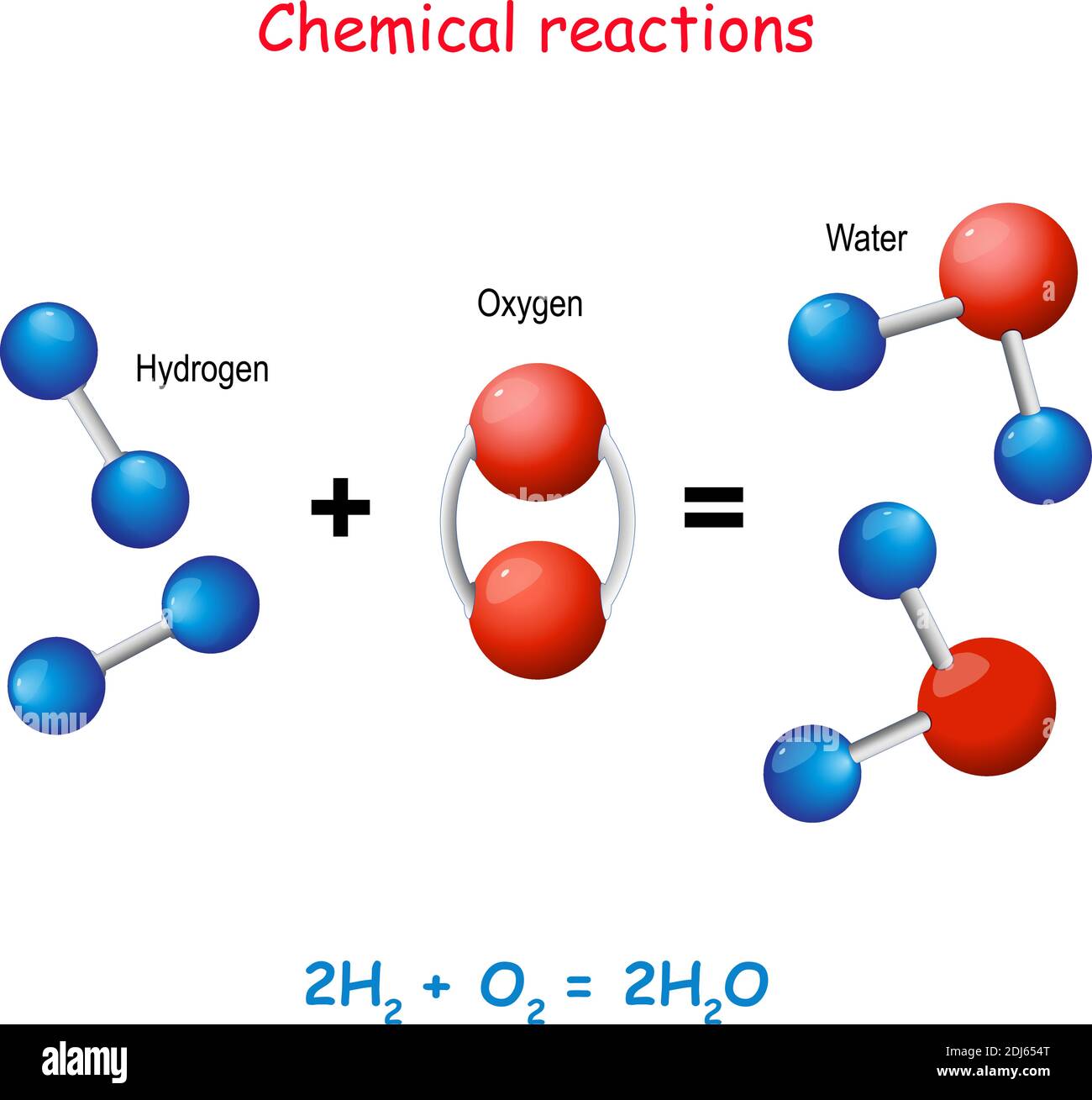
In general, the more reactive the metal, the more rapid the reaction is. Sodium + water → sodium hydroxide + hydrogen For example, sodium reacts rapidly with cold water: When a metal reacts with water, a metal hydroxide and hydrogen are formed. For example, P op S tars C an M ake A bsolutely Z illions I f C hildren S pend G enerously.
#Hydrogens reactivity series
One way to remember the order of metals in the reactivity series is to think of a phrase or sentence, which uses the first letter of each metal in the series. This table summarises some reactions of metals in the reactivity series. the more easily it loses electrons in reactions to form positive ions (cations).In general, the more reactive a metal is: (CISA, 2007) OSHA Process Safety Management (PSM) Standard List No regulatory information available.The reactivity series of metals is a chart showing metals in order of decreasing reactivity. (EPA List of Lists, 2022) CISA Chemical Facility Anti-Terrorism Standards (CFATS) Process Safety Management of Highly Hazardous Chemicals Standard ListĮPA Consolidated List of Lists Regulatory Name Occupational Safety and Health Administration's Cybersecurity and Infrastructure Security Agency's Chemical FacilityĪnd the U.S. Environmental Protection Agency's Title III Consolidated List of CAUTION: When in contact with refrigerated/cryogenic liquids, many materials become brittle and are likely to break without warning. Prevent spreading of vapors through sewers, ventilation systems and confined areas. Use a high-expansion foam if available to reduce vapors. CAUTION: For LNG - Liquefied natural gas ( UN1972), DO NOT apply water, regular or alcohol-resistant foam directly on spill. Do not direct water at spill or source of leak. Avoid allowing water runoff to contact spilled material. Use water spray to reduce vapors or divert vapor cloud drift. If possible, turn leaking containers so that gas escapes rather than liquid. Do not touch or walk through spilled material. All equipment used when handling the product must be grounded.
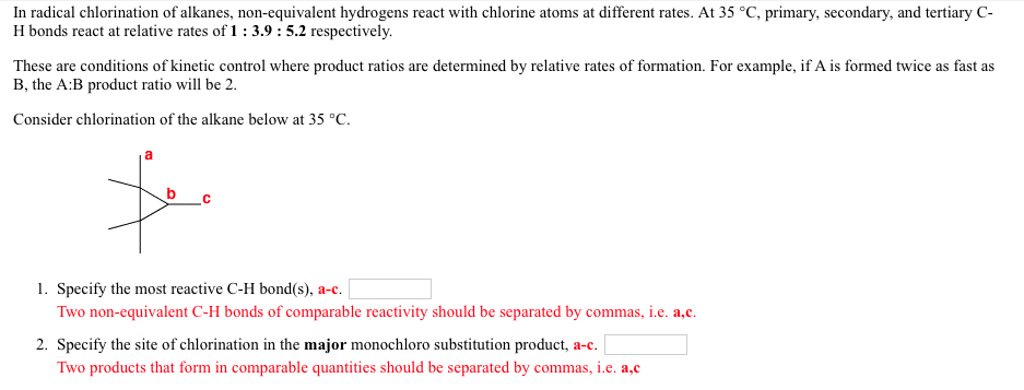
(ERG, 2020)ĮLIMINATE all ignition sources (no smoking, flares, sparks or flames) from immediate area. For massive fire, use unmanned master stream devices or monitor nozzles if this is impossible, withdraw from area and let fire burn.
ALWAYS stay away from tanks engulfed in fire. Withdraw immediately in case of rising sound from venting safety devices or discoloration of tank. Do not direct water at source of leak or safety devices icing may occur. Cool containers with flooding quantities of water until well after fire is out. Use dry chemical or high-expansion foam.įIRE INVOLVING TANKS: Fight fire from maximum distance or use unmanned master stream devices or monitor nozzles. CAUTION: For LNG - Liquefied natural gas ( UN1972) pool fires, DO NOT USE water. If it can be done safely, move undamaged containers away from the area around the fire.
.png)
Use an alternate method of detection (thermal camera, broom handle, etc.). CAUTION: Hydrogen ( UN1049), Deuterium ( UN1957), Hydrogen, refrigerated liquid ( UN1966) and Hydrogen and Methane mixture, compressed ( UN2034) will burn with an invisible flame. Excerpt from ERG Guide 115 :ĭO NOT EXTINGUISH A LEAKING GAS FIRE UNLESS LEAK CAN BE STOPPED.


 0 kommentar(er)
0 kommentar(er)
